

In this tutorial, you will learn about the history of organic chemistry, the importance of organics, and then the difference between organic and inorganic compounds.
Organic compounds are a class of chemicals that are based on carbon. They are found in living organisms and are essential for life, as they are involved in many of the chemical reactions that take place in cells and tissues. They are made up of carbon and other elements, such as hydrogen, oxygen, and nitrogen.
Organic compounds can be divided into two main categories: hydrocarbons, which are compounds made up of only carbon and hydrogen, and compounds with functional groups, which are groups of atoms that are attached to the carbon skeleton of an organic compound and give it specific chemical properties.
Some common examples of organic compounds include sugar, starch, cellulose, oil, carbohydrates, proteins, lipids, nucleic acids, hormones, and DNA. Organic compounds are an important topic in chemistry, and they are studied in many different fields, including biology, medicine, and materials science. They are also found in many everyday products, such as plastics, soaps, and medicines.
Organic chemistry is the study of carbon based compounds that also include hydrogen and heteroatoms, like oxygen, sulfur, halogens, nitrogen, and phosphorous. These elements are all nonmetals. Organic chemists are interested in studying the structure, properties, reactions, and synthesizing more complex organic molecules. Organic chemistry is important to many industries: pharmaceuticals, cosmetics, food stuff, petroleum, etc.
Chemistry has a history in alchemy, but organic chemistry has a history in attempting to understand the relationship between living and non-living things. In 1828, Friedrich Wöhler prepared urea from ammonium cyanate in a laboratory to suggest that an organic compound does not have to be extracted from living things. This work is considered the first organic laboratory synthesis. However, the making of dyes, medicines, and wine from previous centuries are all examples of early organic chemistry and the synthesis of organic material.

Plainly, inorganic compounds are compounds that do not consist of hydrocarbons and heteroatoms. The intramolecular and possible intermolecular interactions between molecules is therefore different; thus, the chemistry of inorganic compounds are different from organics.
In regards to intramolecular forces, the most common type of force keeping organic molecules together is covalent bonds, both polar and non-polar covalent bonds. Inorganic molecules can consist of ionic and covalent bonds. This occasional difference in bonding details how the atoms within these molecules interact with each other differently. Covalent bonds are the result of the sharing of electrons. In comparison, ionic bonds form as a result of an electrostatic attraction between oppositely charged ions as a result of the transfer of ions.
Organic and inorganic compounds can also experience similar intermolecular forces. To begin, both organic and inorganic experience London Dispersion Forces, or a temporary attraction due to molecules being in close proximity. Additionally, both inorganics and organics can experience hydrogen bonding and dipole dipole interactions.
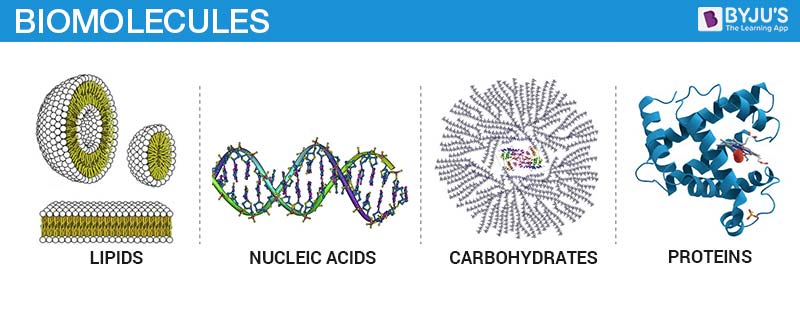
Aside from their importance in industry settings, organic chemicals are also important to biological processes. The four major types of biomolecules, or macromolecules, are lipids, nucleic acids, carbohydrates, and proteins. These are the four types of organics that are necessary for the survival of an organism. For example, DNA and RNA are nucleic acids, so there are organic compounds.
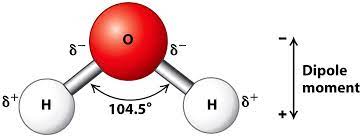
Water molecules experience covalent bonds and are also extremely important to survival of organisms. Despite water’s significance to life, it is not an organic compound. Based on the structure of water, as shown above, it is not an organic compound. Organics are carbon based; water, therefore, is not.


